We discover and develop novel TCR-based therapeutics against solid tumors
Our Science
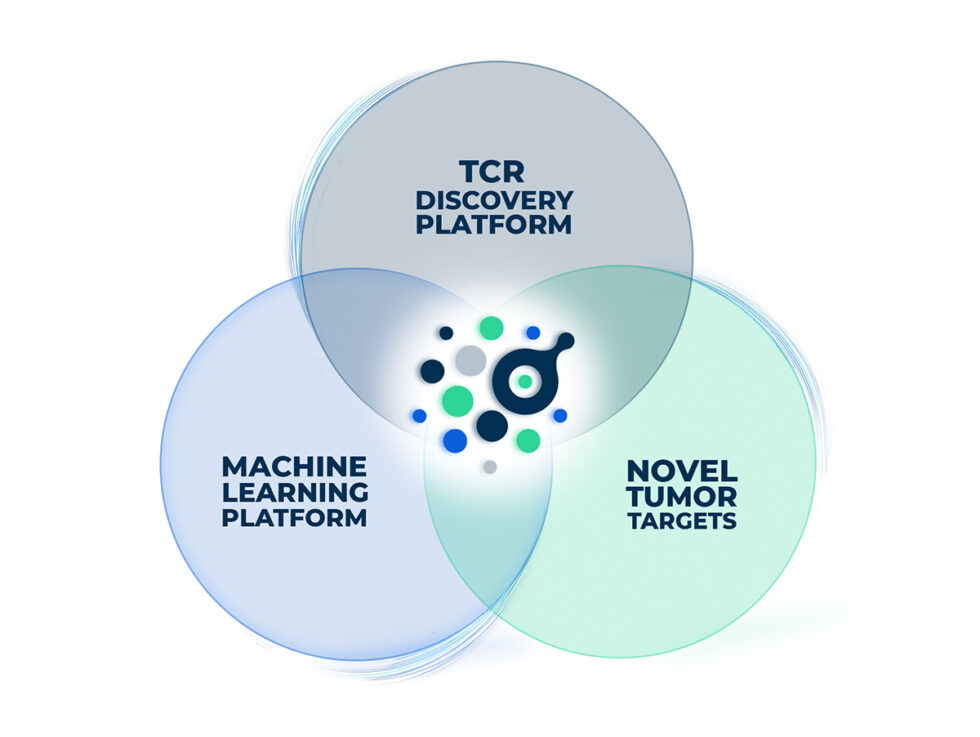
We discover and develop a diverse, risk-balanced portfolio of novel TCRs against solid tumors leveraging our Deep Immunomics and Machine Learning platforms. In combination with novel tumor targets, ImmunoScape is using a multi-pronged approach to develop highly effective and differentiated T cell receptor based therapeutics.
Our Pipeline
ImmunoScape’s discovery engine is continuously generating unique TCR hits and is rapidly moving assets towards clinical candidate nomination. Our TCR-based therapeutics pipeline consists of different cell therapy programs against solid tumors.

Latest Articles
2023 was an exciting year at ImmunoScape, featuring transformative technical progress,...
The collaboration seeks to produce novel off-the-shelf TCR-based bispecific molecules...
MiNK Therapeutics combines its unique, proprietary library of T-cell antigens...
Therapeutics for solid tumors remain in a nascent corner of cancer research despite...
About ImmunoScape
ImmunoScape is a pre-clinical biotechnology company focused on the discovery and development of next-generation TCR-T cell therapies in the field of oncology. We are based in Singapore and in San Diego, US.
